
When an atom in an excited state undergoes a transition to the ground state in a process called decay, it loses energy by emitting a photon whose energy corresponds to the difference in energy between the two states ( Figure 6.11 "The Emission of Light by a Hydrogen Atom in an Excited State").Įquation 6.12 1 λ = − ℛ ( 1 n 1 2 − 1 n 2 2 )Įxcept for the negative sign, this is the same equation that Rydberg obtained experimentally. A hydrogen atom with an electron in an orbit with n > 1 is therefore in an excited state Any arrangement of electrons that is higher in energy than the ground state.: its energy is higher than the energy of the ground state. As n increases, the radius of the orbit increases the electron is farther from the proton, which results in a less stable arrangement with higher potential energy ( Figure 6.10 "The Bohr Model of the Hydrogen Atom"). Because a hydrogen atom with its one electron in this orbit has the lowest possible energy, this is the ground state The most stable arrangement of electrons for an element or a compound., the most stable arrangement for a hydrogen atom. Thus the orbit with n = 1 is the lowest in energy. The latter condition is arbitrarily assigned an energy of zero. The negative sign in Equation 6.9 is a convention indicating that the electron-nucleus pair has a lower energy when they are near each other than when they are infinitely far apart, corresponding to n = ∞. Where ℛ is the Rydberg constant, h is Planck’s constant, c is the speed of light, and n is a positive integer corresponding to the number assigned to the orbit, with n = 1 corresponding to the orbit closest to the nucleus.
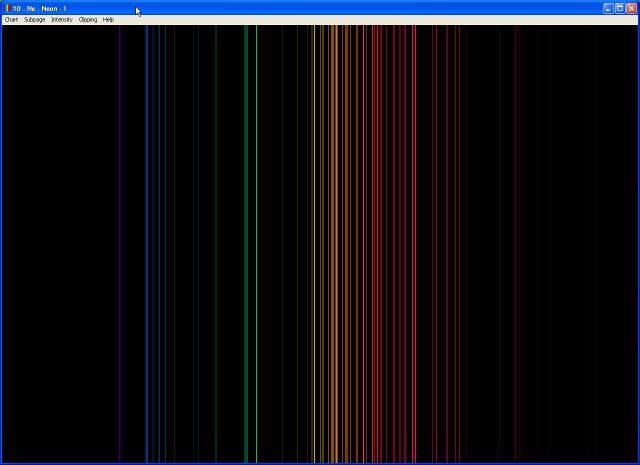
In 1885, a Swiss mathematics teacher, Johann Balmer (1825–1898), showed that the frequencies of the lines observed in the visible region of the spectrum of hydrogen fit a simple equation that can be expressed as follows: If a hydrogen atom could have any value of energy, then a continuous spectrum would have been observed, similar to blackbody radiation. Thus the energy levels of a hydrogen atom had to be quantized in other words, only states that had certain values of energy were possible, or allowed. Part of the explanation is provided by Planck’s equation ( Equation 6.5): the observation of only a few values of λ (or ν) in the line spectrum meant that only a few values of E were possible. Such emission spectra were observed for many other elements in the late 19th century, which presented a major challenge because classical physics was unable to explain them. (b) When the light emitted by a sample of excited hydrogen atoms is split into its component wavelengths by a prism, four characteristic violet, blue, green, and red emission lines can be observed, the most intense of which is at 656 nm. (a) A sample of excited hydrogen atoms emits a characteristic red light.
With sodium, however, we observe a yellow color because the most intense lines in its spectrum are in the yellow portion of the spectrum, at about 589 nm.įigure 6.9 The Emission of Light by Hydrogen Atoms The light emitted by hydrogen atoms is red because, of its four characteristic lines, the most intense line in its spectrum is in the red portion of the visible spectrum, at 656 nm. When the emitted light is passed through a prism, only a few narrow lines, called a line spectrum A spectrum in which light of only a certain wavelength is emitted or absorbed, rather than a continuous range of wavelengths., are seen ( Figure 6.9 "The Emission of Light by Hydrogen Atoms"), rather than a continuous range of colors. Unlike blackbody radiation, the color of the light emitted by the hydrogen atoms does not depend greatly on the temperature of the gas in the tube. For example, when a high-voltage electrical discharge is passed through a sample of hydrogen gas at low pressure, the resulting individual isolated hydrogen atoms caused by the dissociation of H 2 emit a red light. Although objects at high temperature emit a continuous spectrum of electromagnetic radiation ( Figure 6.6 "Relationship between the Temperature of an Object and the Spectrum of Blackbody Radiation It Emits"), a different kind of spectrum is observed when pure samples of individual elements are heated.


 0 kommentar(er)
0 kommentar(er)
